Soda ash is the closest “relative” of baking soda. Housewives are more willing to use the first one in the fight against pollution, the second one in cooking. And if soda ash cannot be used for baking, then it has no equal in bringing order to your home.

- Formula and characteristics of soda ash
- History of discovery
- Difference from other soda
- Benefits and harms of sodium carbonate
- Applications of sodium carbonate
- At home
- In the kitchen
- For cleaning work surfaces, tiles
- When washing clothes
- Boiling laundry
- Removing blockages in pipes
- Cleaning the washing machine and plumbing fixtures
- In gardening
- Treating plants against pests
- Plant rejuvenation
- For lush flowering
- Soap making
- Reducing water hardness
- Where sodium carbonate is not recommended to be used
- Manufacturers and brands
- Where to buy soda ash
- Storing soda ash
- How to make soda ash at home
- Precautionary measures
- How to prepare a solution of soda ash
- For washing clothes in a machine
- For soaking clothes
- For dish washing
- To remove carbon deposits
- To descale the kettle
- For cleaning the stove
- For cleaning the floor
- To remove old paint
- For processing eggs
- For disinfection
Formula and characteristics of soda ash
Soda ash is a chemical compound with the following composition:
- carbon - 1 molecule;
- sodium - 2 molecules;
- oxygen - 3 molecules.
The formula for soda ash is Na2CO3.
Another name for the chemical compound is sodium carbonate or baking soda.
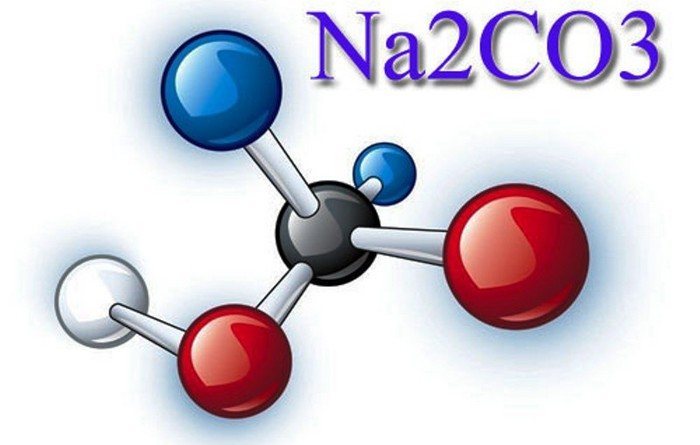
Externally, this compound looks like a white powder.
The main property of this type of soda is water solubility. After reacting with water, it forms an alkaline solution that helps remove stains.
Another property of sodium carbonate is toxicity to various microorganisms: viruses, bacteria, microbes.
History of discovery
Humanity became acquainted with laundry soda a long time ago - 2,000 BC. The ancient Egyptians extracted it from lake waters. Soda was used to melt glass and replaced a detergent.
The word “soda” comes from the phrase “solyanka”. This term denoted a plant that ancient people burned to obtain ash. Then it was heated to high temperatures, i.e. calcified. The resulting substance was soda ash. Soda was mined in this way until the beginning of the 19th century.
The idea of producing laundry soda artificially belongs to the French pharmacist N. Leblanc. He proposed to extract this substance by calcining a mixture of sodium sulfate, coal and crushed chalk. From this composition, sodium carbonate was leached with water, then the solution was evaporated, and pure soda ash was isolated. The released calcium sulfate was considered a production waste.
Subsequently, new methods for extracting laundry soda were developed, which produced much less waste.
In nature, large quantities of sodium carbonate are found in the form of underground ground brines, as well as brine in salt lakes.
Large deposits of this compound are located in Canada, the USA, Mexico, Kenya, and South Africa. There are soda lakes in Western Siberia and Transbaikalia.
Difference from other soda
Laundry soda should be distinguished from baking soda and caustic soda
Baking soda, soda ash and caustic soda are representatives of sodium compounds. But these compounds differ from each other in chemical formula.
Baking soda is a safe product and can be used in everyday life and cooking. It can cause harm to health only if a person ingests a large amount of it.
Soda ash has a high pH value of 11. This compound is a strong alkali and can cause burns and allergies. Can only be used at home. Soda ash has good cleaning properties.
Caustic soda is an alkali that is good at removing contaminants, but can corrode organic matter. The cleansing effect of caustic soda is more pronounced in comparison with food and laundry soda. Caustic soda is a toxic substance.
| Type of soda | Formula | pH | Chemical name | Appearance | Toxicity |
| Food | NAHCO3 | 8 | Sodium bicarbonate | white powder (crystalline) | Non-toxic |
| Calcified | Na2CO3 | 11 | Sodium carbonate | colorless powder (crystalline) | + |
| Caustic | NaOH | 13 | Sodium hydroxide | white scaly mass (hard) | + |
Benefits and harms of sodium carbonate
Soda ash is a beneficial compound and here's why:
- has good disinfectant and cleaning properties;
- helps remove blockages from sewer pipes;
- approved for use for washing clothes, machine or hand;
- effectively removes scale from the internal surfaces of washing machines and dishes;
- can be used to soften hard water;
- Helps fertilize the soil and destroy garden pests.
Despite many advantages, baking soda also has disadvantages:
- at high concentrations in the air it can cause an explosion;
- may cause burns and skin irritation as it creates an aggressive alkaline environment.

Applications of sodium carbonate
Sodium carbonate is widely used in a wide variety of human activities:
- in gardening;
- at home;
- in handicrafts, etc.
This substance is also used to disinfect premises where animals are kept in the textile, automotive and chemical fields.
At home
To eliminate various contaminants in the home, housewives often use baking soda. But laundry handles stubborn stains much more effectively.
In the kitchen
One of the most favorite places for various types of bacteria to accumulate is the kitchen sink. You can wash it clean using sodium carbonate.
Application:
- Pour baking soda into the sink and leave for 15 minutes.
- Walk over the sink with a sponge, especially thoroughly wiping dirty areas.
- Run a sponge over the faucet - after cleaning with white powder, it will begin to shine.
- Rinse off any remaining powder with water.
- Wipe the sink and faucet with a clean, dry cloth to prevent streaks.
If limescale has accumulated in the area around the base of the faucet, the contaminated surface should be sprinkled with laundry soda. Moisten the powder a little with water and let stand for 30 minutes. After the required time, remove the dirt with a sponge and wipe the surface with a dry, clean cloth.
For cleaning work surfaces, tiles
Sodium carbonate is an effective means of removing stains from tiles and kitchen countertops.
Ingredients:
- laundry soda - 3 tbsp. l.;
- water - 1 l.
Application:
- Dissolve baking soda in water.
- Moisten the sponge with the resulting solution and wash the tiles or work surface.
- To remove heavy stains, it is useful to go over them with a sponge several times.
- Wipe the surface with a damp, clean cloth.
When washing clothes
Soda ash can be used for hand washing clothes. But not all fabrics can be washed with this product. Silk, wool and completely synthetic items will not be happy with washing soda - after washing they will become hard and brittle.
Ingredients:
- water - 2.5 l;
- laundry soap (liquid) - 0.5 tbsp.;
- borax - 0.5 tbsp;
- sodium carbonate - 0.5 tbsp.
Application:
- Pour laundry soap into 500 ml of water, heat the solution on the stove, and wait until the soap is completely dissolved.
- Remove the solution from heat without bringing to a boil.
- Add borax and sodium carbonate to the soap mixture.
- Place the solution on the fire again and wait until it boils.
- Cool the mixture and apply for hand washing.
Sodium carbonate, unlike washing powders, does not provoke allergic reactions.
Boiling laundry
Soda ash is also used for disinfection and high-quality bleaching of fabrics.
Ingredients:
- water - 10 l;
- sodium carbonate - 500 g;
- “Whiteness” - 500 ml.
Application:
- Dip the laundry into the resulting solution.
- Boil things for 40-60 minutes, stirring occasionally.
- Allow the laundry to cool, rinse and dry.
Precautionary measures:
- during boiling, it is important not to breathe in the vapors of the soda solution, open the windows in the room;
- Do not boil laundry in copper or aluminum containers: sodium carbonate will react with these metals, and the laundry will become stained;
- Do not wash fabrics with embroidery and items made of delicate materials in this solution;
- White tablecloths, bed linen, and napkins are best bleached in the solution.
Removing blockages in pipes
If the water from the sink in the bathroom or kitchen does not drain well, it is not necessary to invite a plumber. Sodium carbonate can easily fix this problem. It eats away food residues, grease and dirt that are stuck in pipes well
Recipe No. 1.
Ingredients:
- sodium carbonate - 0.5 tbsp;
- vinegar - 0.5 tbsp;
- bleach "Whiteness" - 0.5 tbsp;
- water (boiling water) - 3 l.
Application:
- Pour baking soda into the drain hole.
- Pour bleach and vinegar in there.
- Close the drain hole with a plug and leave for half an hour.
- After the required time, pour boiling water into the hole.
- After 2 minutes, rinse the pipes with running water.
Recipe No. 2.
Ingredients:
- sodium carbonate - 0.5 tbsp;
- hot water - 1l.
Application:
- Pour baking soda into the drain hole.
- Pour boiling water into the pipe.
- Rinse the drain with hot water for 2 minutes.
It is important to protect your respiratory tract with respirators - chlorine vapors are dangerous to health. After clearing the clog, you need to ventilate the room.
Cleaning the washing machine and plumbing fixtures
Sodium carbonate effectively removes urinary stones, soap deposits, rust, and limescale. Therefore, the powder can be used to clean the surfaces of a bathtub, toilet or shower. It is also suitable for cleaning the drum of a washing machine.
Cleaning plumbing.
Ingredients:
- baking soda - 3 tbsp. l.;
- sodium carbonate - 3 tbsp. l.;
- “Whiteness” - 1/3 cup;
- vinegar 9% - 1/3 tbsp.;
- sponge;
- spray.
Application:
- Combine both types of soda in a separate container (not used for cooking).
- Rinse the contaminated surface with cold water.
- Apply the mixture to the surface of the toilet, bathtub or sink for 30 minutes.
- Mix vinegar and bleach and pour into a spray bottle.
- Spray the solution onto contaminated plumbing fixtures over the baking soda mixture.
- Wait for the reaction to complete (the mixture hisses and foams).
- Rinse off the remaining mixture with water.
For cleaning the washing machine.
Soda ash helps eliminate mold from the internal parts of the washing machine and rid the device of an unpleasant odor.
Ingredients:
- sodium carbonate - 250 g;
- water - 1 tbsp.;
- vinegar 9% - 2 tbsp.;
- sponge.
Application:
- Pour soda with water.
- Pour the resulting liquid into the washing machine tray intended for detergent compositions.
- Pour acetic acid into the drum.
- Run a wash program at high temperatures for 3 hours.
In gardening
Sodium carbonate will help the gardener destroy garden pests and make plants bloom more luxuriantly.
Treating plants against pests
Baking soda helps protect garden plants from various diseases. Thanks to this white powder, the gardener can destroy pests that cause diseases.
Powdery mildew.
Plants affected by powdery mildew can be saved using a solution of soda ash.
Ingredients:
- water - 10 l;
- laundry soda - 2 tbsp. l.;
- laundry soap - 400 g.
Application:
- Pour laundry soap and soda into the water.
- Stir the solution thoroughly until the soap and soda dissolve.
- Spray infected plants 1-2 times a week.
The solution is best used in clear, windless weather.
Solution for combating viburnum leaf beetle.
This harmful beetle affects not only the leaves of viburnum, but also other cultivated plants. The abundance of these pests on a tree greatly weakens it and provokes diseases. To combat the leaf beetle, it is recommended to use sodium carbonate.
Ingredients:
- soda ash - 1 tbsp. l.;
- potassium soap - 200g;
- water - 1 bucket.
Application:
- Pour laundry soda and grated potassium soap into the water.
- Mix the mixture thoroughly until a homogeneous solution is obtained.
- Spray the plant from the inside of the leaves with the resulting liquid.
- The solution is applied once a week until the pests are completely destroyed.
Plant rejuvenation
Gardeners use this simple and effective recipe to rejuvenate roses.
Ingredients:
- sodium carbonate - 1 tsp;
- magnesium sulfate - 1 tsp;
- ammonia - 0.5 tsp.
Preparation:
- Pour ammonia into the water, add magnesium sulfate soda.
- Stir until a homogeneous solution is obtained.
- Spray the leaves of plants with the resulting liquid once every 10 days.
For lush flowering
Baking soda is a useful fertilizer for plants that love alkaline soil. Sodium carbonate will make them bloom lushly. It is useful to treat hydrangea and geranium begonias with this solution.
Ingredients:
- laundry soda - 1 tsp;
- water - 5 l.
Preparation and use:
- Dilute baking soda in water and mix the solution thoroughly.
- Water the plants with the resulting liquid during the formation of buds.
Soap making
Sodium carbonate is also used in home crafts. With its help, you can create a soap that is very good at removing dirt from things after working in the garage or in the garden. You can also use it to remove stains from clothes by boiling the laundry.This product will work most effectively on white items when washed at temperatures above 40 C°.
Ingredients:
- water - 10 l:
- vegetable oil - 8 l;
- sodium carbonate - 3 kg;
- table salt - 1.5 tbsp.
Preparation:
- Boil water in a large container, reduce heat and slowly pour in vegetable oil.
- Boil the resulting mixture for 5-10 minutes, stirring continuously.
- Add soda and salt.
- Continue cooking the mixture over low heat, stirring continuously so that it does not burn.
- When all the ingredients are mixed, the mixture will begin to swell. At this time, a soapy substance will appear on the surface of the mixture. This is a signal that the soap is ready and it’s time to pour it into molds.
- Line the bottom of each mold with paper to prevent the soap from sticking.
- When the product has completely cooled, it is placed in a cool, dry place, where the soap is stored for another 4-5 weeks.
The mixture hardens within a few days, that is, much earlier than the specified period. It is not recommended to use soap right away - it will not foam well. The product will acquire all the properties of real soap when it “ripes.”
To make soap colored, you can add green clay, milk, dried seaweed, honey, etc. to the solution.
Reducing water hardness
Baking soda can be used to soften water, which is then used for household needs. You can’t drink this liquid, but you can wash your hands and water the plants.
Ingredients:
- sodium carbonate - 2 tsp;
- water - 10 l.
Application:
- Pour sodium carbonate into water and stir.
- Wait for visible sediment to form.
- Use water for its intended purpose.
Where sodium carbonate is not recommended to be used
Soda ash is not used for the following materials:
- suspended ceilings;
- painted surfaces;
- some types of fabrics: dermantine, suede, wool, silk, leather;
- fiberglass;
- plastic, including window frames, PVC and refrigerator interiors;
- wood, especially polished and varnished;
- acrylic bathtubs;
- laminate
Manufacturers and brands
In Russia, technical soda ash (GOST 5100-85) is produced by several enterprises:
- NPO Chemical Reagents Plant, Arkhangelsk;
- Slavia, Nizhny Novgorod;
- LLC "TD VIKTAN", Ryazan;
- JSC "Bashkir Soda Company";
- Crimean Soda Plant;
- Metakhim, Moscow.
Industrial enterprises produce 2 types of soda ash:
- Grade A is widely used in non-ferrous and ferrous metallurgy and glass production.
- Brand B is used as a component for creating synthetic detergent compositions in the oil, paint and varnish, and paper industries.
Where to buy soda ash
Sodium carbonate is sold in hardware stores, in departments that sell household chemicals.
Most often, the powder is packaged in bags and packs of 400 g, as well as 600 and 800 g.
Storing soda ash
Sodium carbonate is a friable powder without lumps. In special packaging it can be stored for up to 5 years. After opening the container, laundry soda is stored for up to 25 days.
Sodium carbonate actively absorbs moisture from the air. If the package in which it is contained is open, the compound loses its properties and gradually cakes.
After opening the package, it is better to store the soda in a glass container, tightly closing it with a plastic lid. The bank must definitely sign it.
How to make soda ash at home
You don’t have to go to the store to buy sodium carbonate; you can make this powder yourself.
Materials:
- baking soda;
- oven;
- baking dish.
Preparation:
- Preheat the oven to 230 C°.
- Pour the desired amount of baking soda into the baking dish (optional).
- Place the baking sheet with baking soda in the oven for 1 hour.
- During the cooking process, the soda crystals need to be stirred periodically.
- After time has passed, remove the pan from the oven.
- After the soda has cooled, pour it into a clean container and use it for its intended purpose.
Precautionary measures
Soda ash can cause poisoning if it enters the body. Therefore, when using this powder, you must follow the safety rules:
- work with powder only with rubber gloves;
- keep away from medicines, children, food;
- in the process of preparing solutions, strictly observe the proportions;
- If the powder gets into your nose or eyes, be sure to rinse the affected area with water.
How to prepare a solution of soda ash
A few more recipes for solutions based on laundry soda will help the housewife in solving everyday problems.
For washing clothes in a machine
It is permissible to replace washing powder with laundry soda.
Pour 3 tbsp into the detergent compartment. l. soda If the laundry is very dirty, you can add 5 tbsp. l. Some housewives pour the powder directly into the drum.
Using soda and powder together.
Ingredients:
- laundry soda - 100g;
- washing powder is the usual one-time dose.
Application:
- Mix the powder and soda, pour into the powder compartment or drum.
- Wash items as usual at a temperature of at least 40 C°.
For soaking clothes
A simple and affordable method of removing stains from clothes has been used by housewives for several decades in a row.
Ingredients:
- sodium carbonate - 3 tbsp. l.;
- water - 1 bucket;
- laundry soap.
Application:
- Pour baking soda into water to obtain a homogeneous solution.
- Rub the laundry with laundry soap.
- Dip the laundry in a soda solution and soak overnight.
- Place items in the washing machine, set the desired mode at a temperature above 40 C°.
Soaking with vegetable oil.
As a result of this treatment, greasy stains are easily removed from white sheets and napkins.
Ingredients:
- laundry soda - 3 tbsp. l.;
- vegetable oil - 3 tbsp. l.;
- bleach - 3 tbsp. l.;
- washing powder - 3 tbsp. l.;
- water - 1 l.
Preparation:
- Pour all ingredients into hot boiled water and place towels in it.
- Keep the laundry in the water until it cools down.
- Finally, rinse the towels with clean water and start machine washing.
For dish washing
Sodium carbonate effectively cleans household utensils of dirt.
Method number 1.
Ingredients:
- laundry soda - 3 tbsp. l.;
- water - 3 tbsp. l.
Application:
- Mix the ingredients to a paste.
- Distribute the resulting composition over the surface of the dish.
- Let the mixture stand for 5 minutes. Rinse the paste thoroughly with running water.
Method number 2.
Ingredients:
- laundry soda - 3 tbsp. l.;
- water - 1 l.
Preparation:
- Pour soda with water, stir until a homogeneous solution is obtained.
- Place the dishes on which a lot of fat has accumulated in the resulting mixture.
- After 5 minutes, remove and rinse the utensils with running water.
To remove carbon deposits
Sodium carbonate is particularly effective at dissolving carbon deposits from ceramic pans. It is also used to clean enamel dishes.
Method number 1.
Ingredients:
- large capacity (preferably steel);
- sodium carbonate - 1 pack;
- silicate glue - 1 bottle;
- laundry soap - 200 g.
Preparation:
- Place the pans and pots in a large container.
- Pour in water so that it completely covers the dishes.
- Pour silicate glue and a package of soda into the water.
- Pour in grated laundry soap.
- Boil the contents of the container for 20-30 minutes.
- Rinse the items with running water.
This cleaning method is not suitable for utensils made of aluminum and cast iron. Alkali has a destructive effect on these metals.
Method No. 2.
Ingredients:
- Sodium carbonate - 5 tbsp.;
- Acetic acid - 0.5 tbsp.
Use:
- Pour acetic acid into the pan, leave for half an hour.
- Pour soda into the pan, heat over low heat for 10 minutes.
- Remove the mixture with running water.
To descale a kettle
You can use washing soda to clean a teapot from dirt and remove scale from the bottom of the kettle in which water is boiled on the stove.
We clean the teapot from dirt.
- Ingredients:
washing soda - 4 tbsp.;
- vinegar essence - 1 tbsp.;
- large container.
- Application:
- Immerse the teapot in the container.
Pour in water, add soda and vinegar essence.
- The saucepan in which the teapot will be processed should be placed in the sink, since foam will form during operation.
When the foam subsides, put the container on the stove and boil for half an hour.
- After the required amount of time, take the kettle out and rinse with running water.
Descaling.
You can clean the bottom and walls of the kettle from scale using the following recipe.
Ingredients:
sodium carbonate - 1 tsp;
- water - 1 l.
- Preparation:
- Make a water-soda solution.
- Pour the resulting liquid into a teapot, put it on the stove, bring to a boil.
Rinse the teapot thoroughly with running water.
- To clean the stovePreparation:
- Place the pans and pots in a large container.
- Pour in water so that it completely covers the dishes.
- Pour silicate glue and a package of soda into the water.
Boil the contents of the container for 20-30 minutes.
Rinse the items with running water.
- This cleaning method is not suitable for utensils made of aluminum and cast iron. Alkali has a destructive effect on these metals.
- Method No. 2.
- Ingredients:
Sodium carbonate - 5 tbsp.;
- Acetic acid - 0.5 tbsp.
- Use:
- Pour acetic acid into the pan, leave for half an hour.
- Pour soda into the pan, heat over low heat for 10 minutes.
Remove the mixture with running water.
To descale a kettle
You can use washing soda to clean a teapot from dirt and remove scale from the bottom of the kettle in which water is boiled on the stove.
- We clean the teapot from dirt.
- Ingredients:
- washing soda - 4 tbsp.;
vinegar essence - 1 tbsp.;
large container.
Application:
- Immerse the teapot in the container.
- Pour in water, add soda and vinegar essence.
- The saucepan in which the teapot will be processed should be placed in the sink, since foam will form during operation.
- When the foam subsides, put the container on the stove and boil for half an hour.
After the required amount of time, take the kettle out and rinse with running water.
- Descaling.
- You can clean the bottom and walls of the kettle from scale using the following recipe.
Ingredients:
- sodium carbonate - 1 tsp;
- water - 1 l.
- Preparation:
- Make a water-soda solution.

Pour the resulting liquid into a teapot, put it on the stove, bring to a boil.
Rinse the teapot thoroughly with running water.
To clean the stovePreparation:
Place pans and pots in a large container.
- Pour water so that it completely covers the dishes.
- Pour silicate glue and a package of soda into the water.
- Pour in grated laundry soap.
- Boil the contents of the container for 20-30 minutes.
Rinse the products with running water.
- This cleaning method is not suitable for utensils made of aluminum and cast iron. Alkali has a destructive effect on these metals.
- Method number 2.
- Ingredients:
- sodium carbonate - 5 tbsp. l.;
- acetic acid - 0.5 tbsp.
- Application:
Pour acetic acid into the pan and leave for half an hour.
- Pour baking soda into a frying pan and heat over low heat for 10 minutes.
- Remove the mixture with running water.
- To descale the kettle
- Using laundry soda, you can clean the teapot from dirt and remove scale from the bottom of the kettle in which water is boiled on the stove.
Clean the teapot from dirt.
- Ingredients:
- laundry soda - 4 tbsp. l.;
- vinegar essence - 1 tbsp.;
- large container.
- Application:
- Immerse the teapot in the container.
- Pour in water, add soda and vinegar essence.
- The pan in which the teapot will be processed must be placed in the sink, since foam will form during operation.
- When the foam subsides, place the container on the stove and boil for half an hour.
After the required amount of time, remove the kettle and rinse with running water.
Descaling.
You can clean the bottom and walls of the kettle from scale using the following recipe.
Ingredients:
sodium carbonate - 1 tsp;
water - 1 l.
- Preparation:
- Make a water-soda solution.
- Pour the resulting liquid into the kettle, put it on the stove, and bring to a boil.
Rinse the kettle thoroughly with running water.
- For cleaning the stove
- Ovens, stoves and stoves will shine clean after using the glue-soda mixture.
Method number 1.
- Ingredients:
- sodium carbonate - 3 tbsp. l.;
- silicate glue - 1 tbsp. l.;
water - 1 l and 1 tbsp.;
- washing powder - 0.5 tbsp. l.
- Preparation:
Prepare the solution: pour glue into 2 liters of water, add soda.









